Acute lymphoblastic leukemia (ALL) is a rare form of cancer that commonly affects children, mostly under the age of five years. In the search for new therapeutic options, researchers at Vetmeduni Vienna funded by the FWF SFB ‘JAK-STAT & Chromatin Landscapes’ have discovered cyclin-dependent kinase 8 (CDK8) as part of the disease process and have developed a novel drug treatment line that is pioneering for future cancer therapies.
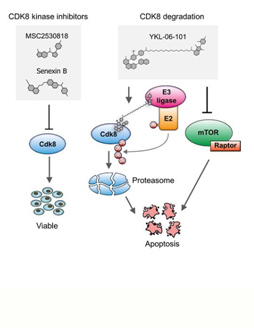
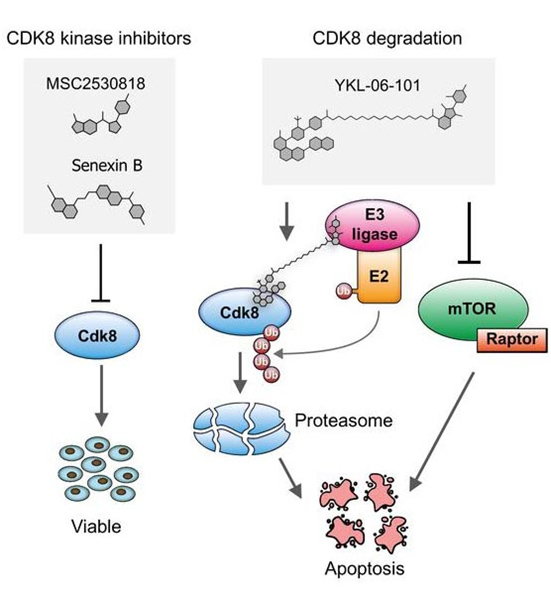
Using leukemia mouse models, first author Ingeborg Menzl from the Institute of Pharmacology and Toxicology at Vetmeduni Vienna and her colleagues demonstrated that CDK8-deficient leukemia cells show an increase in cell death. Notably, this function of CDK8 in ALL is independent of enzymatic activity, which means that conventional kinase inhibitors are ineffective. The search for CDK8 interaction partners revealed a previously unknown link to mTOR signaling in cancer cells.
Dual degrader – a therapy line with combined effect. In collaboration with the research team of Nathanael Gray from the Harvard Medical School, the researchers used a new generation of drugs that do not block enzymatic activity but induce the degradation of proteins (called PROTACs). Using a newly synthesized PROTAC mTOR signaling was blocked while simultaneously CDK8 was degraded. With this concept of a dual degrader, the researchers are pioneering for future cancer therapies.
Publication in Nature Communications
Ingeborg Menzl, Tinghu Zhang, Angelika Berger-Becvar, Reinhard Grausenburger, Gerwin Heller, Michaela Prchal-Murphy, Leo Edlinger, Vanessa M. Knab, Iris Z. Uras, Eva Grundschober, Karin Bauer, Mareike Roth, Anna Skucha, Yao Liu, John M. Hatcher, Yanke Liang, Nicholas P. Kwiatkowski, Daniela Fux, Andrea Hoelbl-Kovacic, Stefan Kubicek, Junia V. Melo, Peter Valent, Thomas Weichhart, Florian Grebien, Johannes Zuber, Nathanael S. Gray and Veronika Sexl;
Doi: https://doi.org/10.1038/s41467-019-12656-x