Innate immunity to viral infection is achieved by a group of polypeptide mediators, the interferons (IFN). By binding to cell surface receptors they initiate signal transduction via Janus kinases (JAK) that the STATs target, a group of transcription factors. STATs combine to form a transcription factor, ISGF3, that activates transcription of a large number of IFN-induced genes (ISG) encoding antiviral proteins and establishing an antiviral state.
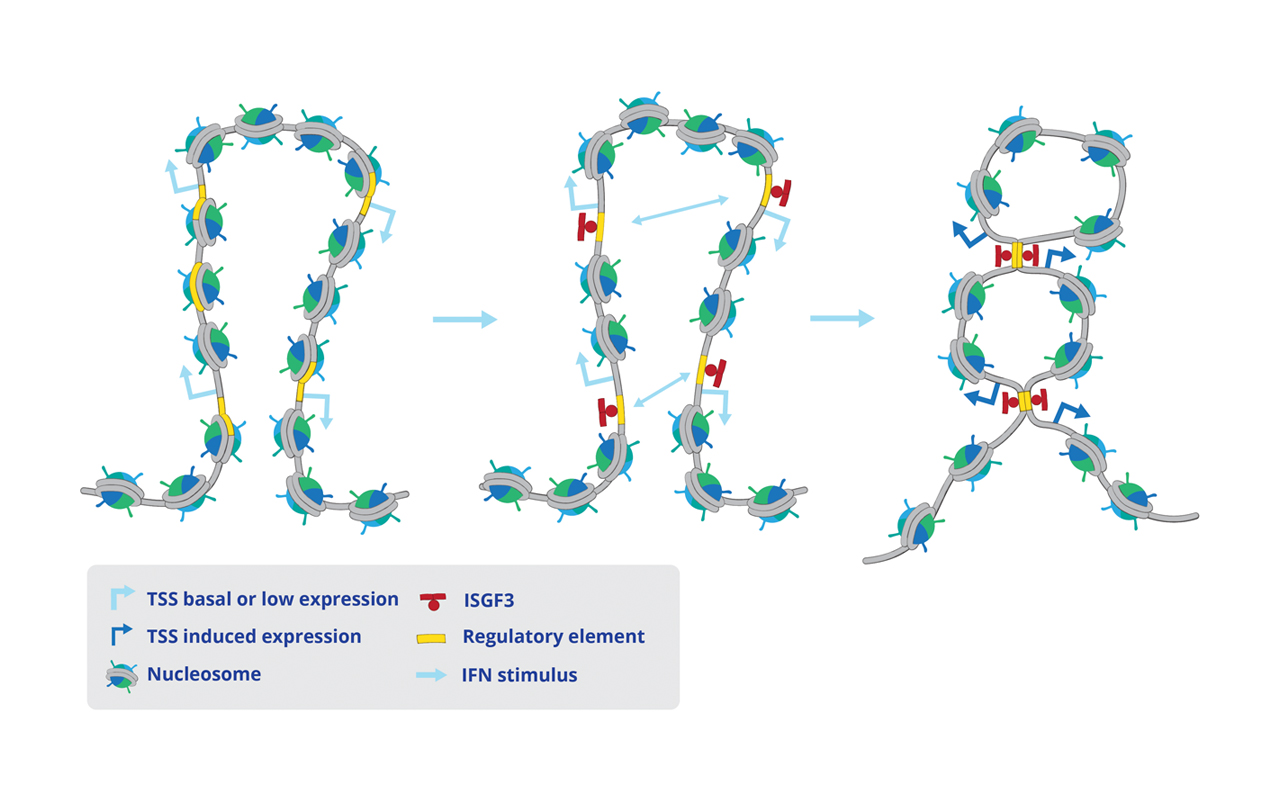
We show that the transcription of antiviral genes includes important changes of chromatin structure (see below). First, ISGF3 binds to the control region of ISG to induce a rearrangement of nucleosomes that creates maximal accessibility of the promoter (below middle panel). Second, ISG that are arranged in chromosomal clusters change their chromatin loop structure to increase the interaction of regulatory elements (below right panel). The molecular model emerging from the study posits that interaction in the 3-dimensional space creates regulatory hubs with the ability to influence the expression of several clustered genes simultaneously. This may help to both coordinate, accelerate and strengthen the establishment of the antiviral state.
IFN stimulus alters the 3-dimensional chromatin architecture at antiviral gene clusters
Published in iScience
Ekaterini Platanitis, Sthephan Gruener, Aarathy Ravi Sundar Jose Geetha, Laura Boccuni, Alexander Vogt, Maria Novatchkova, Andreas Sommer, Iros Barozzi, Mathias Müller, Thomas Decker
Interferons reshape the 3D conformation and accessibility of macrophage chromatin