Acute myeloid leukemia (AML) is an aggressive form of blood cancer that affects children and adults. In cases with particularly poor prognosis, this cancer is triggered by oncogenic fusion proteins, the formation of which involves the Nucleoporin 98 (NUP98) gene. A study published in the journal Blood results from a collaborative effort, including the groups of Richard Moriggl and Veronika Sexl of the Vetmeduni Vienna, and introduces a new therapeutic approach to fight this disease.
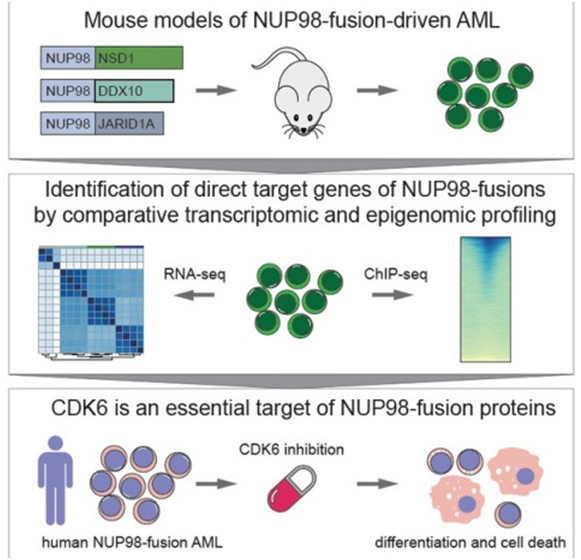
Genetic rearrangements, in which the NUP98 gene is involved, are rare genetic events that occur repeatedly in AML patients and are associated with a particularly poor prognosis - especially if this process occurs in children and adolescents. In a cooperation that included the Institute of Biochemistry and the Institute of Pharmacology at the Vetmeduni Vienna, researchers have for the first time identified the genes that are activated directly by NUP98 fusion proteins.
The authors developed novel mouse models that mimic the rare blood cancer AML, which included NUP98-fusion proteins. By integrating chromatin occupancy profiles of NUP98-fusion proteins with transcriptome profiling they discovered that NUP98-fusion proteins directly regulate leukemia-associated gene expression programs. Among these is the CDK6 protein, for which molecular inhibitors were already approved for clinical usage to treat other types of cancer. The authors then showed that treatment with CDK6 inhibitors significantly improved the survival of the test animals. Further clinical studies are now required to confirm the effectiveness of targeted CDK6 inhibition in patients suffering from AML.
Johannes Schmöllerl, Inês Amorim Monteiro Barbosa, Thomas Eder, Tania Brandstoetter, Luisa Schmidt, Barbara Maurer, Selina Troester, Ha Thi Thanh Pham, Mohanty Sagarajit, Jessica Ebner, Gabriele Manhart, Ezgi Aslan, Stefan Terlecki-Zaniewicz, Christa Van der Veen, Gregor Hoermann, Nicolas Duployez, Arnaud Petit, Helene Lapillonne, Alexandre Puissant, Raphael Itzykson, Richard Moriggl, Michael Heuser, Roland Meisel, Peter Valent, Veronika Sexl, Johannes Zuber and Florian Grebien
Doi: https://doi.org/10.1182/blood.2019003267