Vetmeduni Vienna, January 25th 2021:
Extension of “Monarchies and Hierarchies in Shaping Chromatin Landscapes” Special Research Programme funded by Austrian Science Fund FWF
https://www.vetmeduni.ac.at/en/infoservice/press-releases/presseinformationen-2021/extension-of-monarchies-and-hierarchies-in-shaping-chromatin-landscapes-special-research-programme/
Second funding period (2021-2025) for SFB F61 approved in the FWF Board meeting from 23rd to 25th of November 2020.
Summary of the Research Program
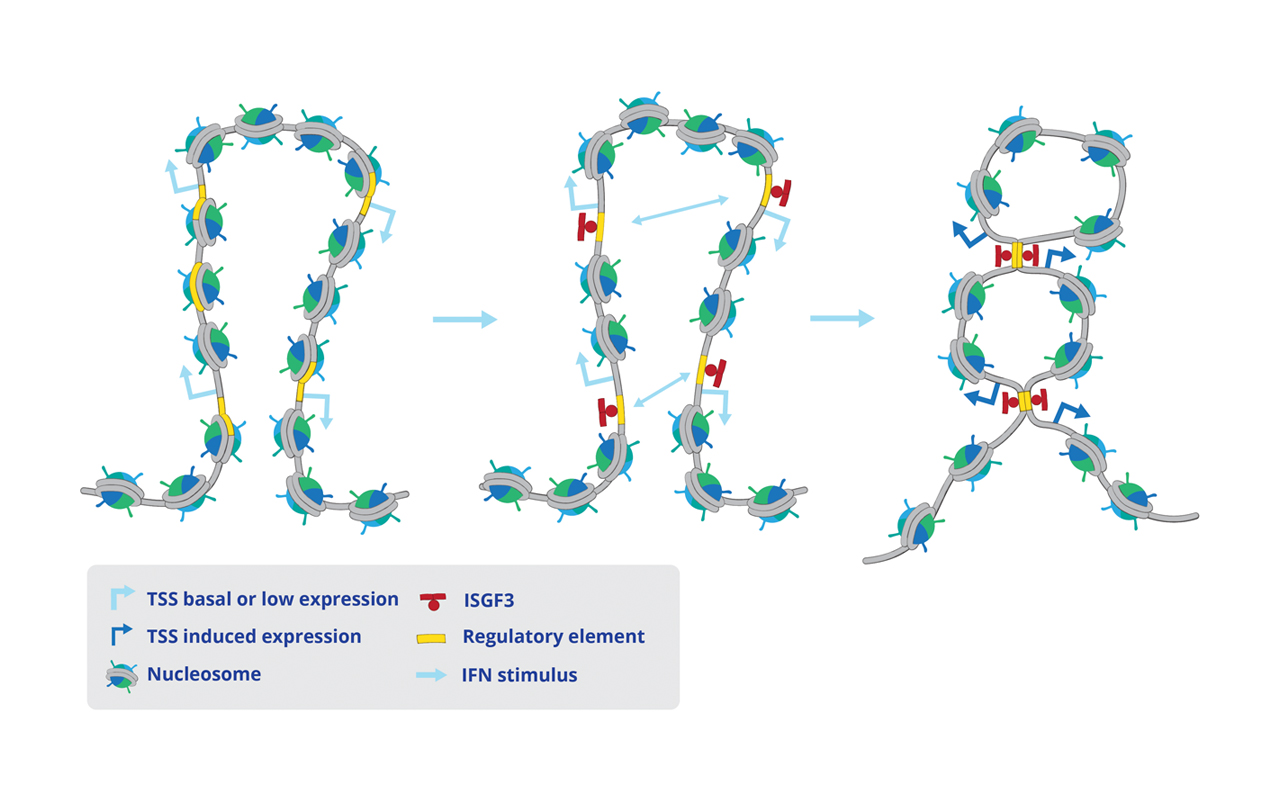
The STAT proteins are transcription factors with a central role in cell homeostasis, survival and differentiation. They are activated by the JAK kinases, including TYK2. Dysregulated STATs cause immune- or inflammation-related, metabolic and tumorigenic diseases but how STATs interact with chromatin is unknown. We have compiled a map of chromatin activity for all wildtype STATs, TYK2, oncogenic STAT5B and a kinase-inactive TYK2 mutant in primary immune cells and in structural cells under homeostatic, cytokine-induced and cell-transforming conditions and plan to use it to determine how JAK-STAT exerts its manifold effects.
The SFB groups hypothesise
- STATs and TYK2 cause chromatin remodeling in non-hematopoietic cells, defining their identity and shaping the interface of immune cells and stromal cells in homeostasis and disease (Christoph Bock, Mathias Müller, Birgit Strobl with all other consortium members)
- Homeostatic macrophages use STAT2/IRF9 to prime themselves for activation (Thomas Decker, Sylvia Knapp and Christoph Bock); signals from STAT1,3,5 drive the exit from and the return to homeostasis (Sylvia Knapp, Mathias Müller, Birgit Strobl, Thomas Decker and Christoph Bock)
- STAT5A and STAT5B are not equivalent but drive distinct developmental programs in haematopoietic and leukaemic cells (Veronika Sexl, Heidi Neubauer and Christoph Bock)
- The chromatin signatures of haematopoietic cancers are shaped by oncogenic STAT5, oncogenic STAT3 or a STAT3-CDK6 complex (Veronika Sexl, Heidi Neubauer and Christoph Bock)
- TYK2 determines cell fate by regulating both transcriptional and post-transcriptional processes (Birgit Strobl, Mathias Müller and Christoph Bock with Thomas Decker).
Much of the current work on signal transduction in disease conditions (e.g. during infection, transformation or drug treatment) is based on an outdated understanding of the homeostatic healthy condition. By providing a fine-scale and cell-specific definition, our work will cause a comprehensive (re-)evaluation of the early stages of perturbations and the return to homeostasis. The approach is completely novel and will revolutionize our understanding of cellular memory and the progression/resolution of disease.