Acute lymphoblastic leukemia (ALL) is a rare cancer that affects mostly affects children. In the search for new therapeutic options, researchers at Vetmeduni Vienna have now discovered a new mechanism of disease development and proposed a completely new treatment - a pioneering work for future cancer therapies. The study has just been published in Nature Communications.
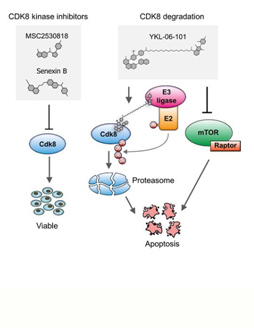
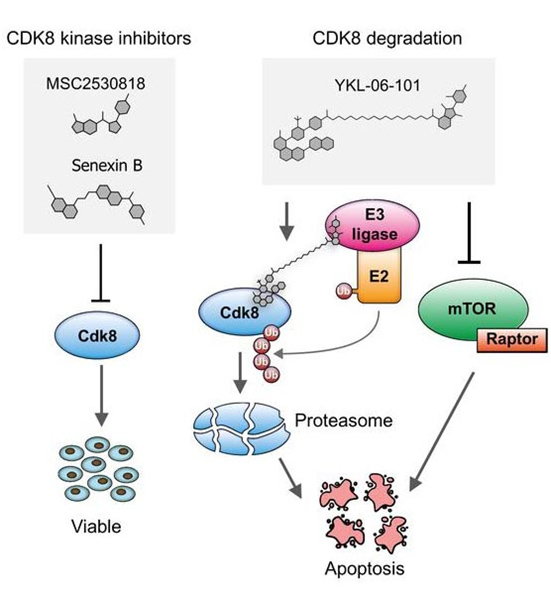
Cyclin-dependent kinases (CDKs) are frequently deregulated in cancer and represent promising drug targets. The research team of Veronika Sexl at the Vetmeduni Vienna - in collaboration with the research team of Nathanael Gray from Harvard Medical School (USA) - focused on CDK8 in the search for new therapeutic routes for ALL. The reason for this is that tumorigenic cells are dependent on CDK8 function, while healthy cells are not. This opens up a therapeutic window by targeting CDK8: healthy cells are spared while cancer cells will be affected.
The research team was able to show that leukemia cells that lose CDK8 in leukemia mouse models significantly enhance disease latency and prevents disease maintenance. Furthermore, CDK8-depleted cancer cells are highly sensitive to mTOR inhibitors, a previously unknown connection. Thus, the authors have synthesized a small molecule (YKL-06-101) that combines mTOR inhibition and degradation of CDK8, and induces cell death in human leukemic cells. This represents a new therapeutic line in drug development: a dual degrader drug is sufficient to break down a molecule - CDK8 - and at the same time enzymatically block a signalling pathway. They propose that by affecting both simultaneously a potential therapeutic strategy for the treatment of ALL patients might be developed.
Published in Nature Communications
Ingeborg Menzl, Tinghu Zhang, Angelika Berger-Becvar, Reinhard Grausenburger, Gerwin Heller, Michaela Prchal-Murphy, Leo Edlinger, Vanessa M. Knab, Iris Z. Uras, Eva Grundschober, Karin Bauer, Mareike Roth, Anna Skucha, Yao Liu, John M. Hatcher, Yanke Liang, Nicholas P. Kwiatkowski, Daniela Fux, Andrea Hoelbl-Kovacic, Stefan Kubicek, Junia V. Melo, Peter Valent , Thomas Weichhart, Florian Grebien, Johannes Zuber, Nathanael S. Gray and Veronika Sexl
Doi: https://doi.org/10.1038/s41467-019-12656-x